iStent infinite® Important Safety Information
Indication for Use
The iStent infinite® Trabecular Micro-Bypass System Model iS3 is an implantable device intended to reduce the intraocular pressure (IOP) of the eye. It is indicated for use in adult patients with primary open-angle glaucoma in whom previous medical and surgical treatment has failed.
Contraindications
The iStent infinite is contraindicated in eyes with angle-closure glaucoma where the angle has not been surgically opened, acute traumatic, malignant, active uveitic, or active neovascular glaucoma, discernible congenital anomalies of the anterior chamber (AC) angle, retrobulbar tumor, thyroid eye disease, or Sturge-Weber Syndrome or any other type of condition that may cause elevated episcleral venous pressure.
Warnings
Gonioscopy should be performed prior to surgery to exclude congenital anomalies of the angle, PAS, rubeosis, or conditions that would prohibit adequate visualization that could lead to improper placement of the stent and pose a hazard.
MRI Information
The iStent infinite is MR-Conditional, i.e., the device is safe for use in a specified MR environment under specified conditions; please see Directions for Use (DFU) label for details.
Precautions
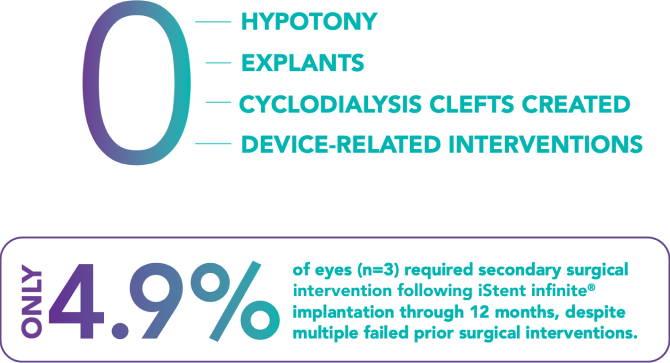
The surgeon should monitor the patient postoperatively for proper maintenance of IOP. Three out of 61 participants (4.9%) in the pivotal clinical trial were phakic. Therefore, there is insufficient evidence to determine whether the clinical performance of the device may be different in those who are phakic versus in those who are pseudophakic.
Adverse Events
The most common postoperative adverse events reported in the iStent infinite pivotal trial included IOP increase ≥ 10 mmHg vs. baseline IOP (8.2%), loss of BSCVA ≥ 2 lines (11.5%), ocular surface disease (11.5%), perioperative inflammation (6.6%) and visual field loss ≥ 2.5 dB (6.6%).
Caution
Federal law restricts this device to sale by, or on the order of, a physician. Please see DFU for a complete list of contraindications, warnings, precautions, and adverse events.