Solutions for Healthcare Providers
iStent inject® W
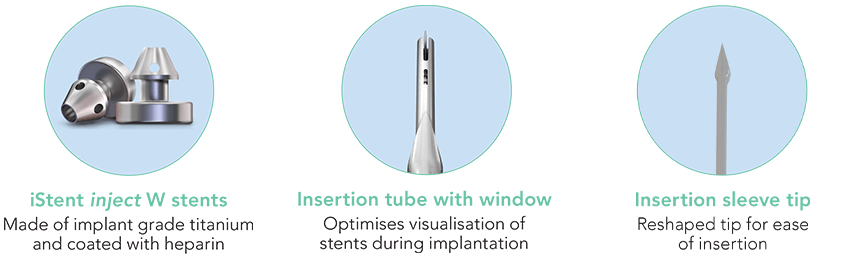
iStent inject® W is intended to reduce intraocular pressure safely and effectively in patients diagnosed with primary open-angle glaucoma, pseudo-exfoliative glaucoma, or pigmentary glaucoma. And it’s precision-engineered to optimise stent visualisation and placement, enhance procedural predictability, and increase confidence.
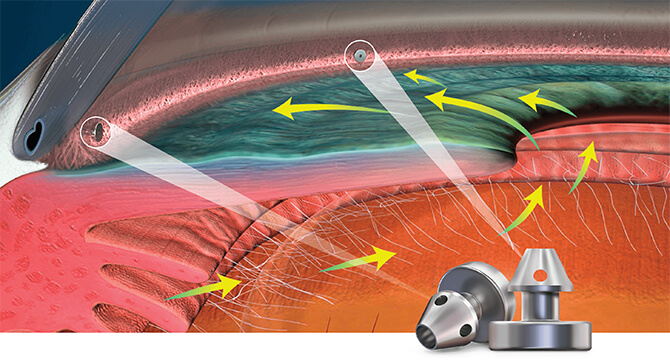
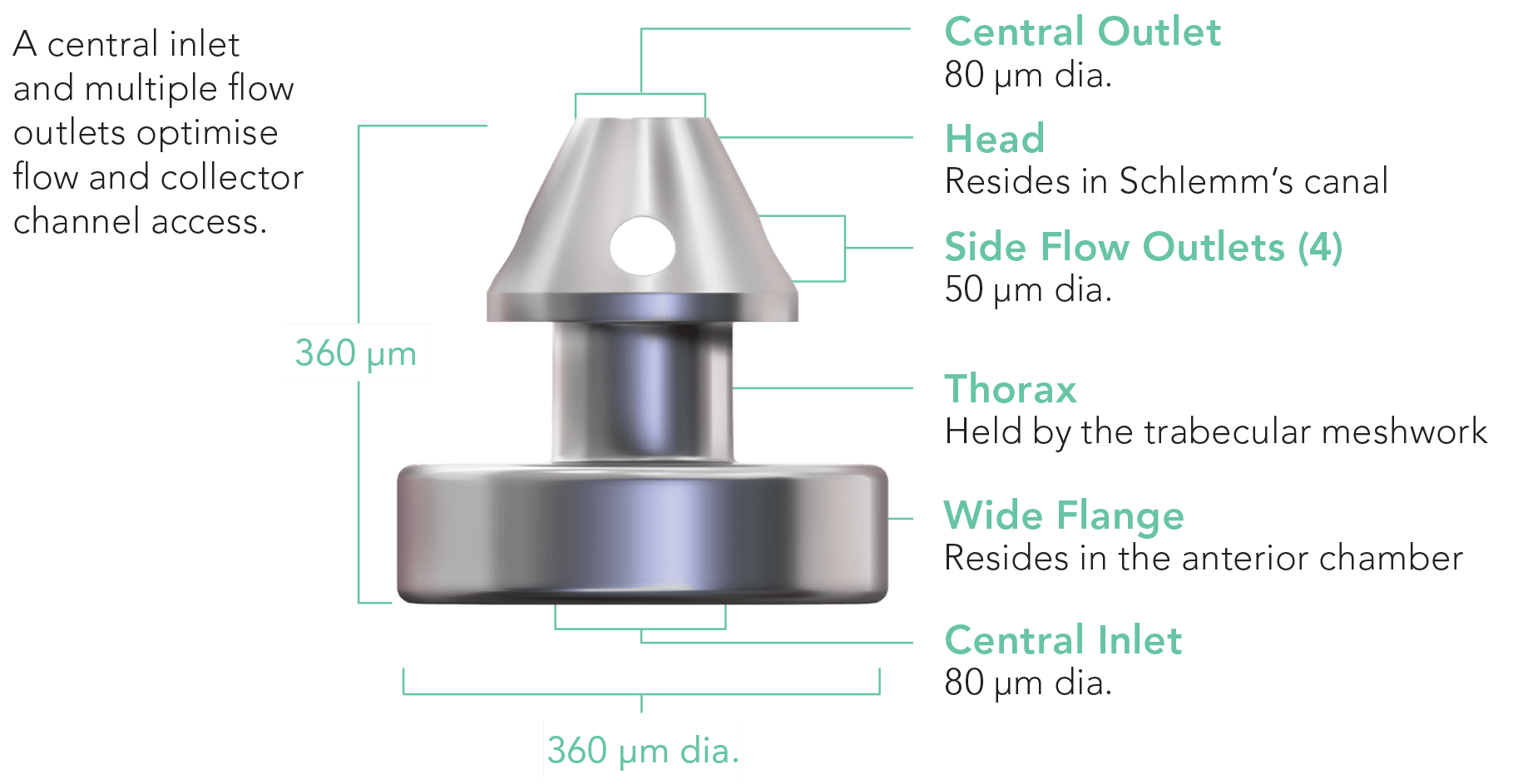
Mechanism of Action

Delivering two preloaded trabecular micro-bypass stents with a single entry, iStent inject® W is designed to optimise the benefits of Trabecular Micro-Bypass surgery. The iStent inject® W device is designed to improve aqueous outflow through the natural physiological outflow pathway. When the device is implanted in the trabecular meshwork, it is designed to stent open that section of the ocular anatomy to allow aqueous humor to flow from the anterior chamber into Schlemm’s canal. A natural episcleral back pressure of 8-11 mm Hg in the trabecular meshwork reduces the risk of hypotony.1

Watch How It Works
The iStent inject® W Procedure
iStent inject® W is inserted ab interno through a microincision, which may be the phaco incision if performed in conjunction with cataract surgery, and can be performed under topical anaesthesia.
Tilt the patient’s head up to 25° away from the surgeon and tilt the microscope up to 35° toward the surgeon to obtain proper anterior chamber angle viewing
Adjust the microscope (10-12x) and locate the trabecular meshwork
 |
Deepen the anterior chamber with a cohesive viscoelastic as needed |
 |
Enter the eye with the inserter through a clear corneal incision and advance past the pupillary margin |
 |
Place the trocar through the centre or slightly anterior portion of the trabecular meshwork and into the back wall of Schlemm’s canal |
 |
Dimple the tissue enough to see a “V” when pressing on trabecular meshwork |
 |
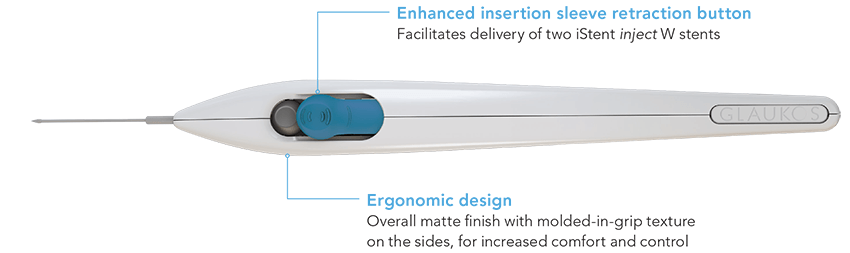
Squeeze the stent delivery button, but keep your finger on the stent delivery button while pulling back from the implanted stent |
 |
Move 2 to 3 clock hours away and implant the second stent using the same technique |
Focused on Excellent Outcomes
See the latest clinical data around iStent inject® W patient outcomes.
We’re here to answer questions and provide any additional information you may need.
"*" indicates required fields
- Hengerer FH. Personal Experience with Second-Generation Trabecular Micro-Bypass Stents in Combination with Cataract Surgery in Patients with Glaucoma: 3-Year Follow-up. ASCRS 2018 Presentation.
- Samuelson TW. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. March 2011;118(3):459-467.
- Voskanyan L, García-Feijoó J, Belda J, Fea A, Jünemann A, Baudouin C. “Prospective, Unmasked Evaluation of the iStent inject System for Open-Angle Glaucoma: Synergy Trial”. Adv Ther 2014; 31:189-201.
