Clinical Data
The INTEGRITY Study1
The interim 6-month data suggests iStent infinite® is statistically superior or has clinically superior outcomes compared to Hydrus Microstent.


Study Overview
-

LARGEST HEAD-TO-HEAD, PROSPECTIVE RCT
- Largest prospective RCT comparing trabecular bypass devices in patients with POAG; 180 eyes in total (91 iStent infinite®, 89 Hydrus Microstent)
-

180 EYES
- 180 eyes in total (91 iStent infinite®, 89 Hydrus Microstent)
-

7 SITES | 13 SURGEONS
- 7 sites with 13 experienced iStent infinite® and Hydrus Microstent surgeons
-

MEDICATION WASHOUT
- Per protocol, medication washout deployed at baseline and predetermined timepoints (6 months, 12 months, 24 months)
-

STANDALONE IMPLANTATION
- Standalone implantation of iStent infinite® or Hydrus Microstent
-

PROSPECTIVELY DEFINED PRIMARY AND SECONDARY OUTCOMES
- Mean change in Month 12 MDIOP from baseline
- Unmedicated MDIOP reduction >20% from baseline at Month 6 with no pertinent safety findings*
- Safety parameters: intraoperative/postoperative complications, corrected visual acuity, slit-lamp and fundus examinations, gonioscopy, visual field testing, and adverse events
RCT=randomized controlled trial.
POAG=primary open-angle glaucoma.
MDIOP=mean diurnal intraocular pressure.
Month 6 Findings
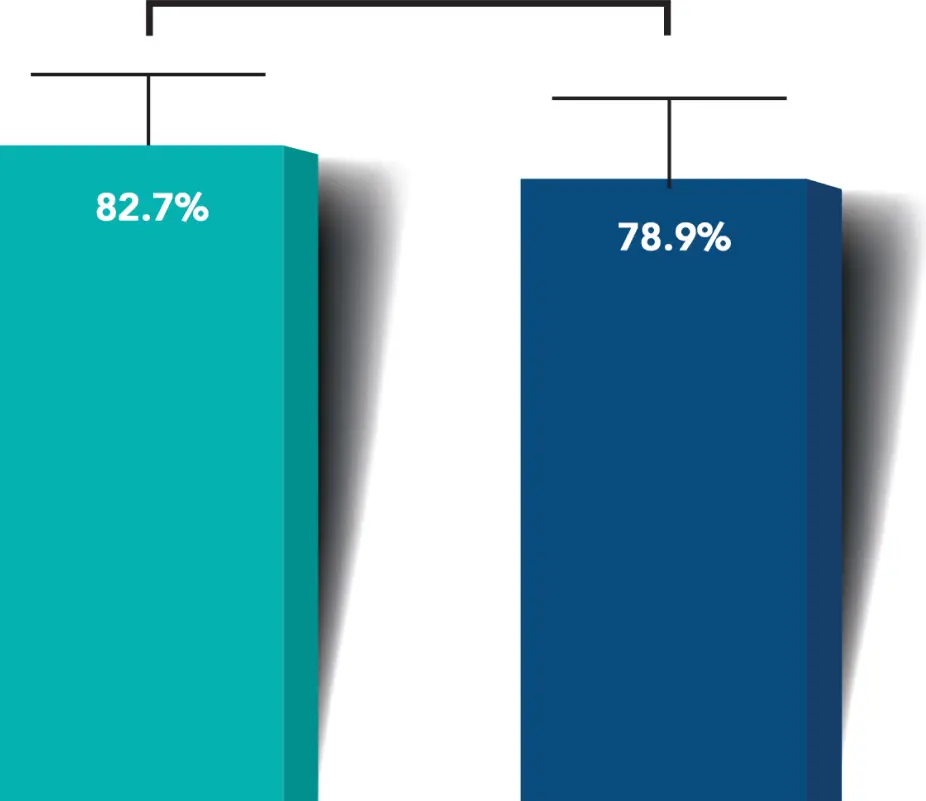
Proportion of Eyes with Month 6 Unmedicated MDIOP Reduction from Baseline ≥ 20%
(Excluding all intercurrent events)

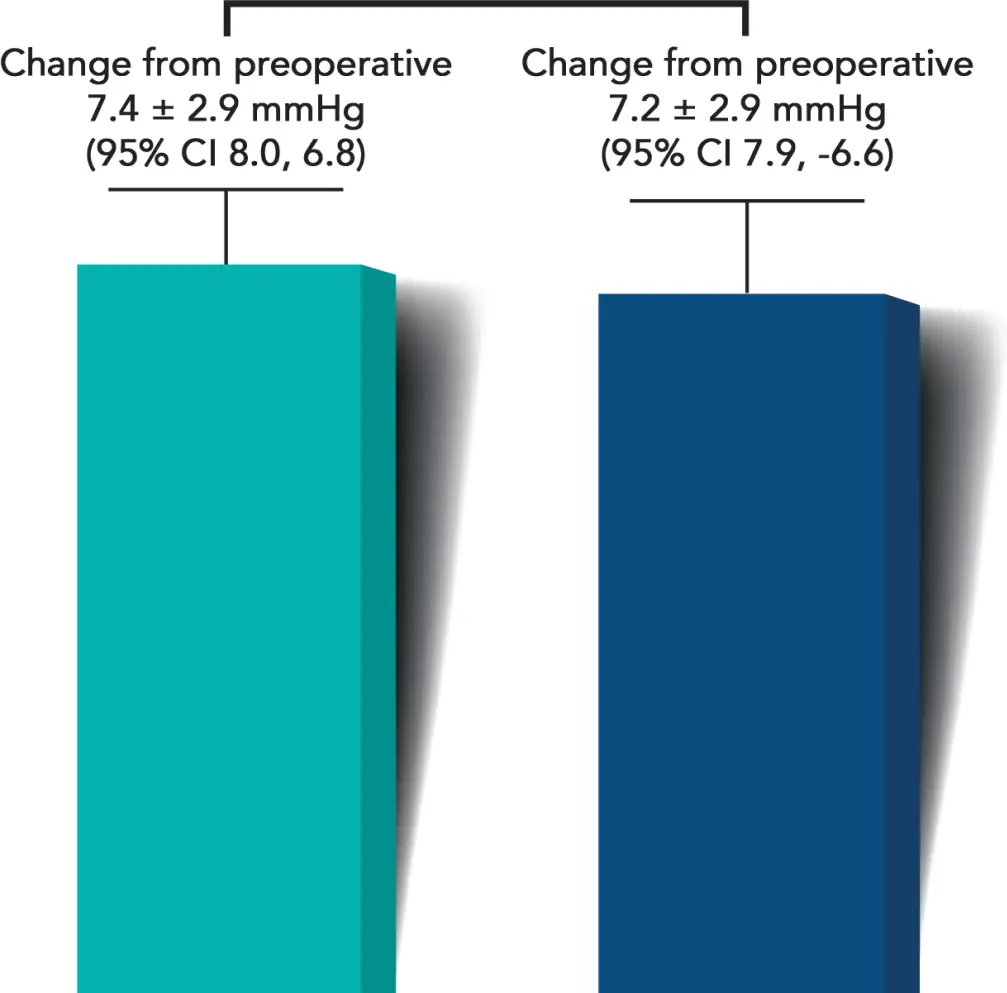
Month 6 Unmedicated MDIOP Reduction from Baseline
(Excluding all intercurrent events)

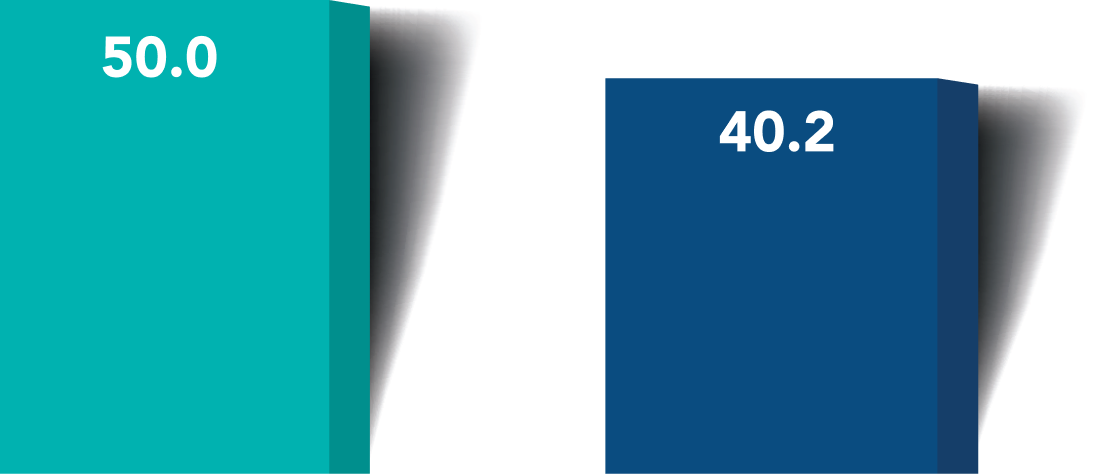
More iStent infinite® eyes had a ≥20% reduction in unmedicated diurnal IOP from baseline with no surgical complications*
≥20% reduction
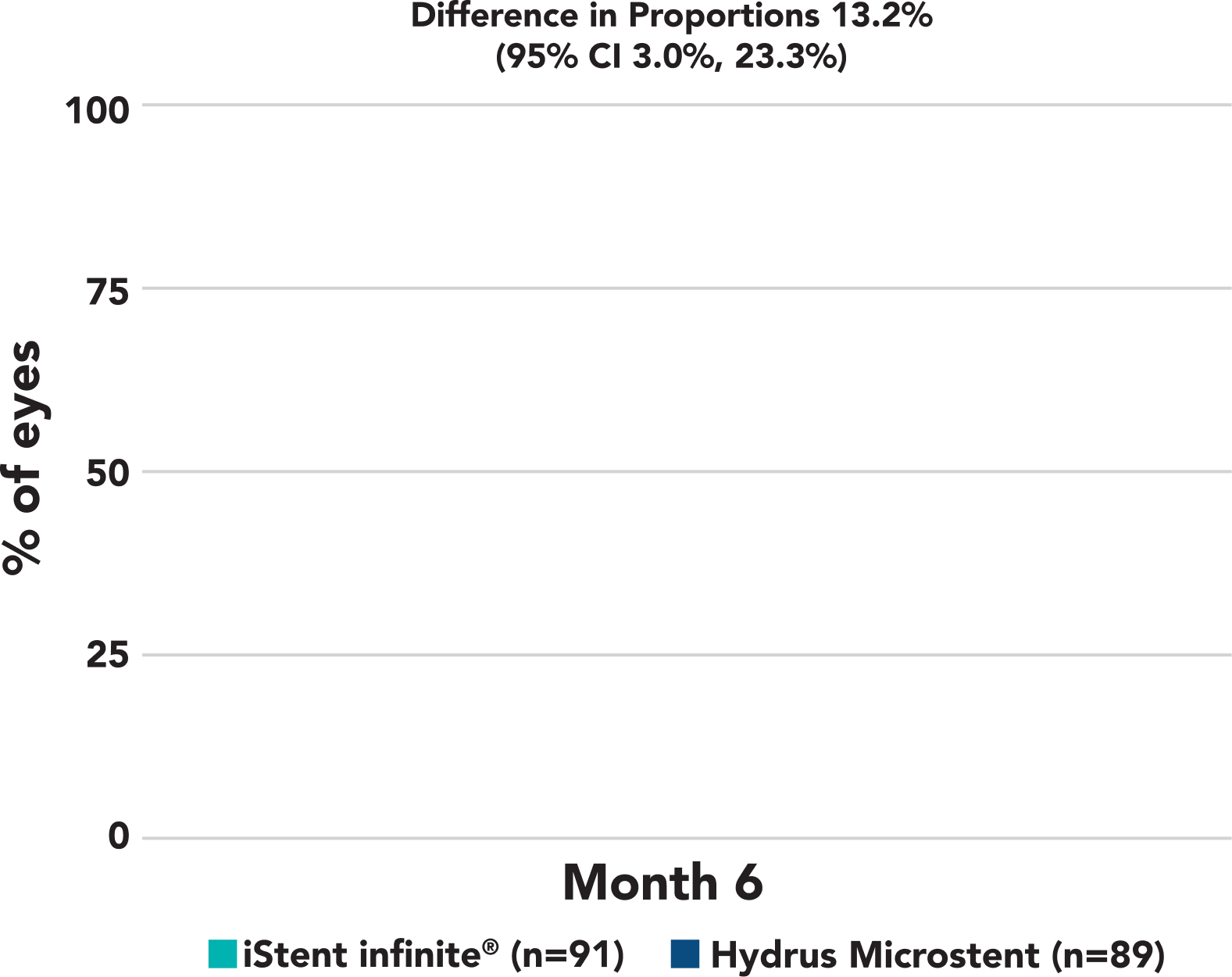
More iStent infinite® eyes had ≥30% reduction in unmedicated diurnal IOP (P > 0.05)
≥30% reduction

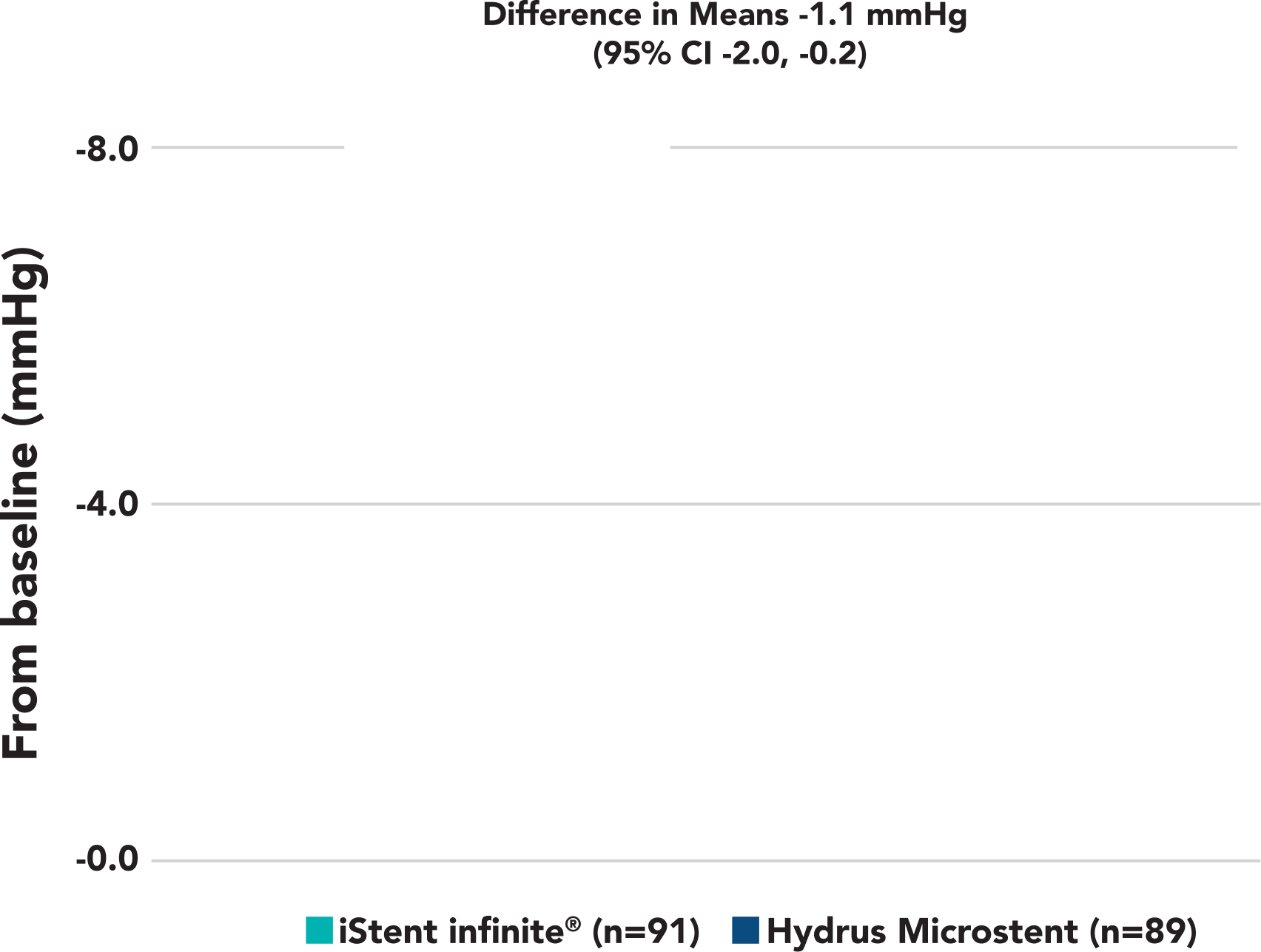
A statistically significant and clinically meaningful reduction in diurnal IOP from baseline was observed in iStent infinite® eyes
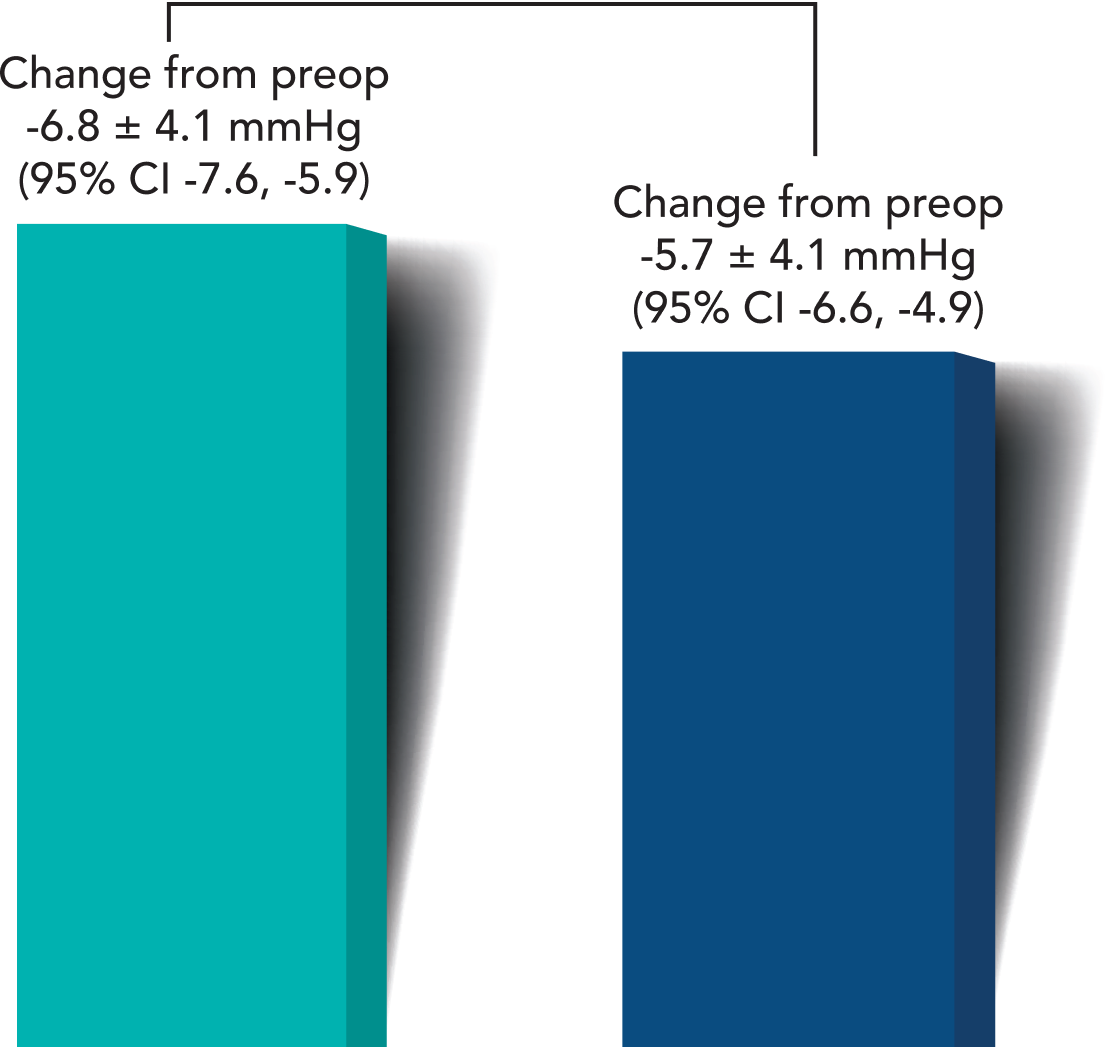
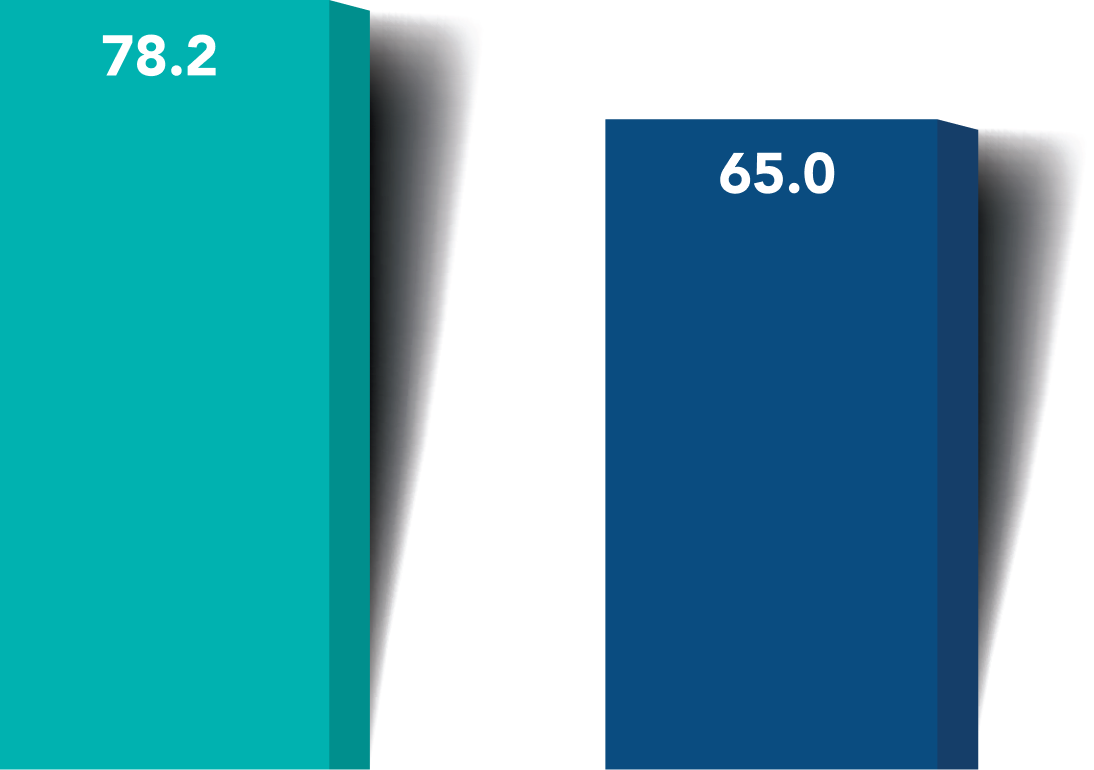
A greater proportion of iStent infinite® eyes had lower unmedicated post-op mean diurnal IOPs

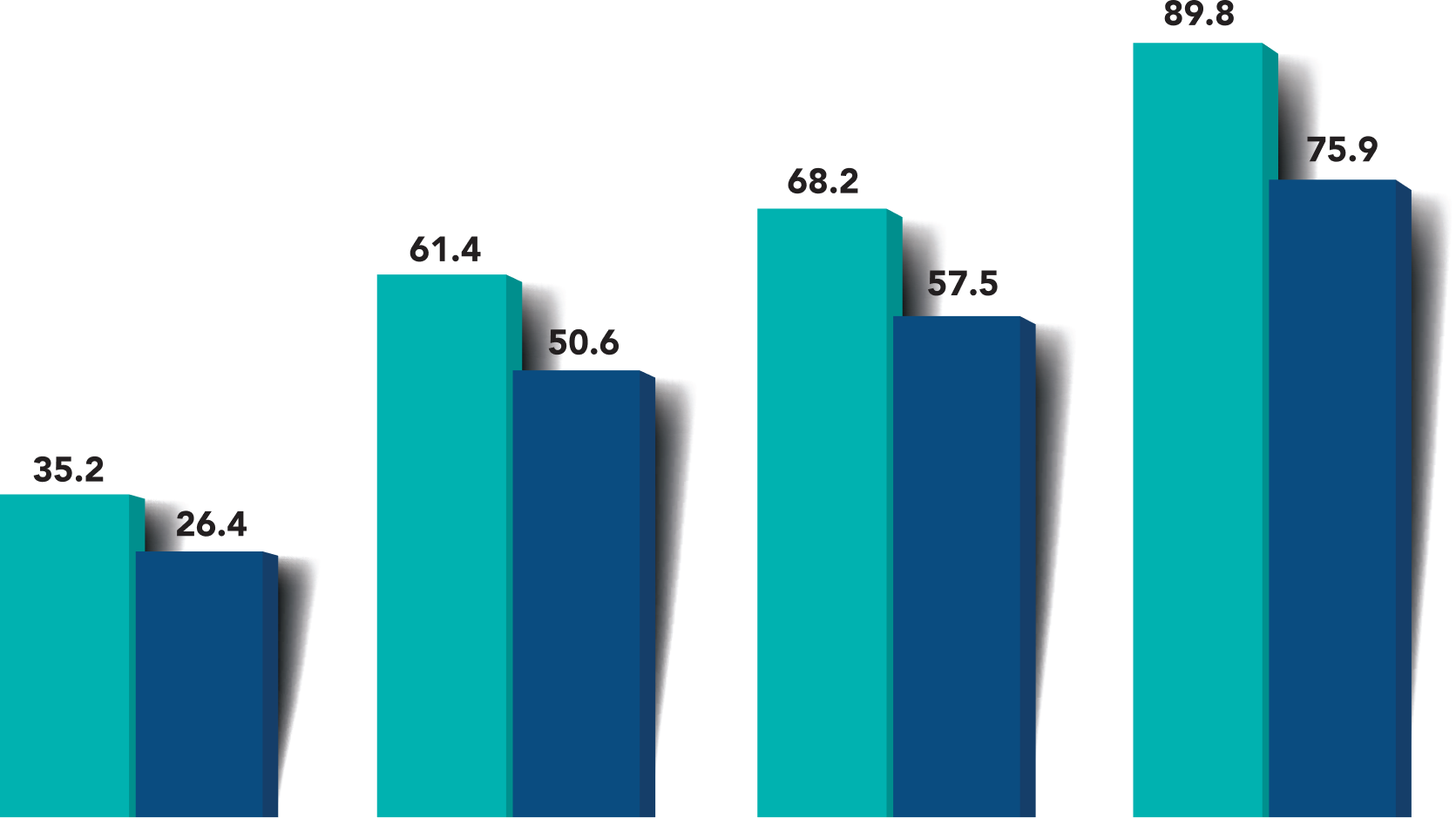
Fewer intraoperative and postoperative adverse events were observed with iStent infinite®
Improper anatomical placement
iStent infinite®
Hydrus Microstent
Peripheral anterior synechiae (>1mm)
iStent infinite®
Hydrus Microstent
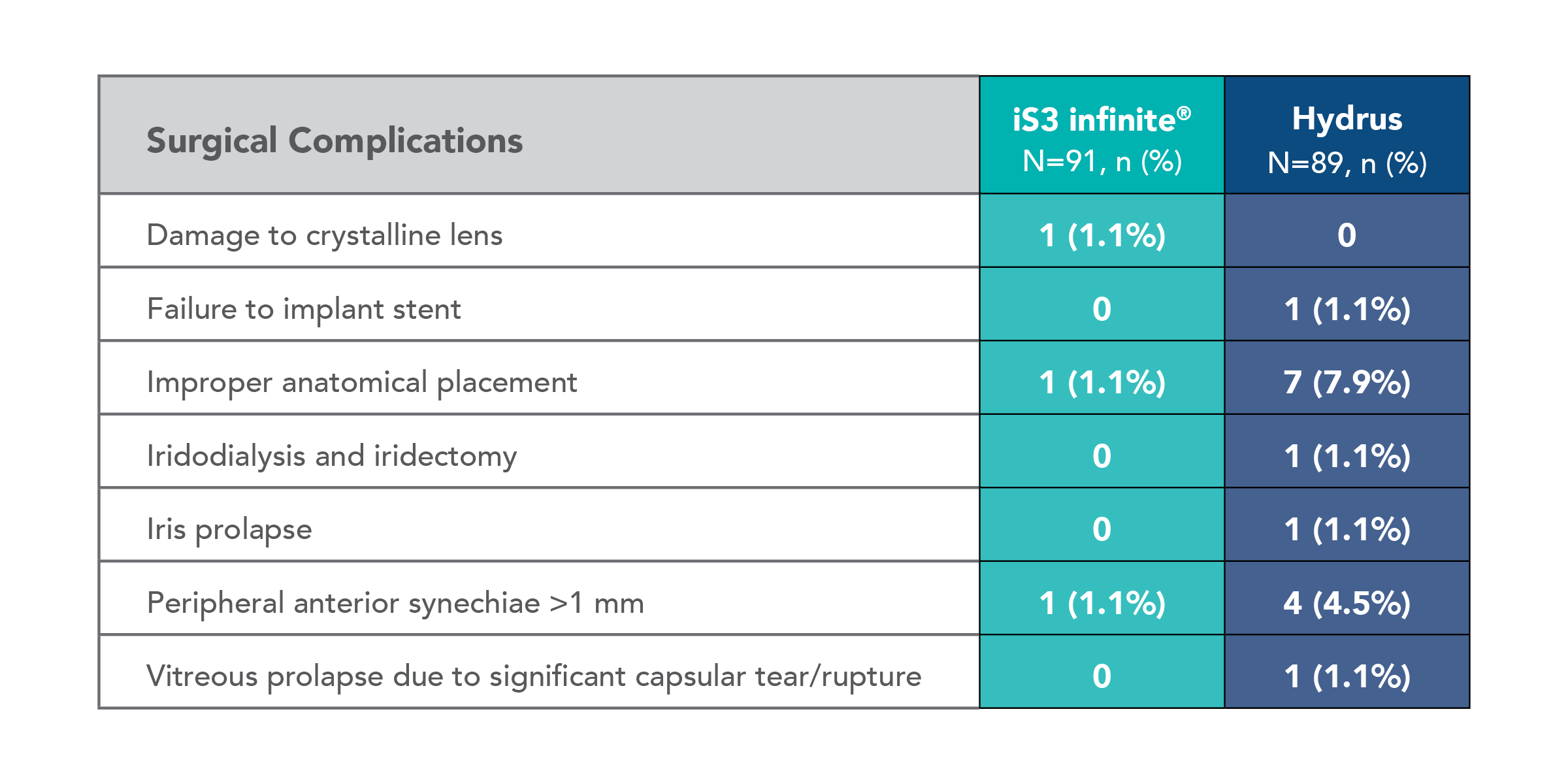
*Surgical complications include damage to crystalline lens, failure to implant stent, improper anatomical placement, iridodialysis and iridectomy, iris prolapse, peripheral anterior synechiae > 1 mm, vitreous prolapse due to significant capsular tear/rupture
Reference
- Glaukos Data on File.
Trademarks are the property of their respective owners.
Hydrus is a registered trademark of Alcon, Inc. Image of Hydrus from https://www.myalcon.com/professional/cataract-surgery/hydrus-microstent/
