iLink®
Not all corneal cross-linking procedures are the same.
Lead the Way With iLink®
When it comes to referring patients with progressive keratoconus for treatment, iLink® is the only FDA-approved corneal cross-linking procedure.

Only iLink® offers:
-
Confidence
in your keratoconus treatment recommendation with proven efficacy and data
-
Coverage
for over 95% of commercially insured lives
-
Consideration
for the health of your patients and your peace of mind when making referrals
FDA Approved
If it’s not iLink®,
it’s not FDA approved

Don’t settle for less. There are several unapproved cross-linking procedures that healthcare providers might recommend or use without your knowing. These unapproved drugs and devices can put your patients’ safety, as well as your and your practice’s reputation, at risk.
Peschke Trade GmbH Import Alerts
The FDA has issued import alerts that include Peschke cross-linking devices and riboflavin. These products are illegal for importation and sale in the United States unless they are for a clinical investigation that is part of an FDA-approved investigational new drug (IND) application.

Not all cross-linking procedures are the same
Using any drug and device combination for corneal cross-linking other than iLink® is not just off-label,* it’s unapproved.†
The following chart compares the benefits of iLink® with the risks of unapproved cross-linking. Keep scrolling to see more unfold.
| FDA-Approved iLink® Corneal Cross-Linking | Unapproved Corneal Cross-linking |
|---|---|
|
Approved by the FDA with pivotal clinical trial data and proven efficacy and safety |
Safety and efficacy data have not been reviewed by the FDA, making the procedure unapproved |
|
Coverage is available for over 95% of commercially insured lives1 |
Not covered by insurance—billing insurance for an unapproved procedure may be illegal or considered fraud1 |
|
Patients may be eligible for copay assistance through the Photrexa® Patient Savings Program |
Associated with greater patient out-of-pocket costs upwards of $6500 |
|
Incorporates rigorously tested and controlled Photrexa® (riboflavin 5’-phosphate ophthalmic solution) and Photrexa® Viscous (riboflavin 5'-phosphate in 20% dextran ophthalmic solution) riboflavin solutions1 |
Uses unapproved compounded riboflavin and/or riboflavin from Peschke Trade GmbH that is subject to an FDA import alert1 |
|
OMIC, the largest liability insurance carrier for ophthalmic providers, only covers FDA-approved procedures for commercial use. iLink® is the only FDA-approved cross-linking procedure for progressive keratoconus, and it incorporates the KXL® system, Photrexa®, and Photrexa® Viscous2 |
OMIC advises policyholders that the commercial use of unapproved products may include risks they do not cover2 |
|
Offers patients peace of mind knowing their cross-linking procedure is approved by the FDA |
Patients should be notified of the unapproved status of the procedure and that there is an FDA-approved option available2 |
|
Safety and efficacy are supported by pivotal trial data and numerous clinical studies that are detailed on ClinicalTrials.gov |
Should be done with American IRB oversight and an FDA-approved IND application.2 Physician-sponsored studies (with compounded riboflavin) being listed on ClinicalTrials.gov does not ensure that the study is part of an FDA-approved IND |
|
Photrexa® and Photrexa® Viscous are manufactured under FDA requirements of good manufacturing practice and are not compounded |
The FDA does not verify the safety, effectiveness, or quality of unapproved compounded drugs before they are marketed. Compounded drugs may not be subject to current good manufacturing practice requirements3 |
|
Performed epithelium-off, allowing for Photrexa® and Photrexa® Viscous riboflavin solutions to permeate into the cornea and form strong collagen cross-links1 |
Currently, there are no riboflavin solutions approved by the FDA for epithelium-on procedures |
|
The KXL® system is the only FDA-approved corneal cross-linking device |
Unapproved devices may be modified or illegally imported with no postmarket surveillance1 |
|
Adverse events are documented in the iLink® Important Safety Information |
Do not require reporting on adverse events |
As a Glaukos procedure, iLink® offers confidence and consideration for the safety and peace of mind of your patients. | |
OMIC=Ophthalmic Mutual Insurance Company; IND=investigational new drug; IRB=institutional review board.
*Off-label means an FDA-approved product is being used for an unapproved use.
†Unapproved means that the FDA has not approved the product, determined the drug is safe and effective, or provided any labeling with information that can help patients avoid serious adverse events.

Articles and videos on Unapproved Cross-Linking
Read published articles and watch videos from your peers about the risks of unapproved cross-linking.

Your progressive keratoconus patients rely on you to make a sound referral. Learn more from experts in optometry, ophthalmology and law who delve into unapproved cross-linking topics that matter, including: failed cases, legal and financial issues and critical differences between FDA-approved iLink® and other unapproved cross-linking drugs and devices.

The word is out and insurers have listened
The word is out and insurers have listened
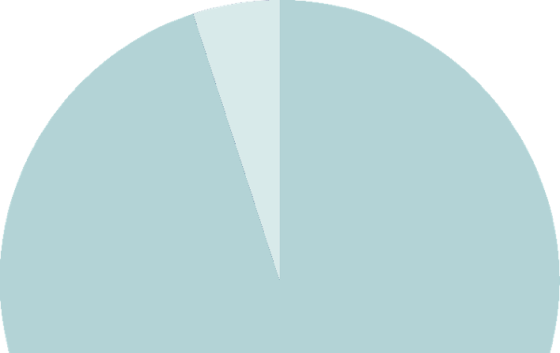
The medical necessity of iLink® is widely recognized for the treatment of progressive keratoconus. As a result, the majority of insured patients in the United States are covered for the procedure.
Over 95%
of commercially insured lives are eligible for insurance coverage for iLink®.
GPS (Glaukos Patient Services)
Provider Assistance
The GPS (Glaukos Patient Services) is connecting providers with the reimbursement support they need to ensure patients can get treated with the FDA-approved iLink® cross-linking procedure.
Refer for iLink® today
Choosing a referral partner who values proven patient outcomes as much as you do has never been easier. iLinkExpert.com connects referring physicians with trusted specialists who offer the FDA-approved iLink® procedure.
References
- Glaukos Data On File. 2020.
- I have patients with post-refractive ectasia who might benefit from corneal collagen cross-linking with riboflavin (CXL/C3-R). Will OMIC cover this treatment? Ophthalmic Mutual Insurance Company. Updated April 22, 2019. Accessed May 21, 2021. https://www.omic.com/policyholder/i-have-patients-with-post-refractive-ectasia-who-might-benefit-from-corneal-collagen-crosslinking-with-riboflavin-c3-rcxl-will-omic-cover-this-treatment.
- Compounding and the FDA: Questions and answers. US Food and Drug Administration. Accessed May 21, 2021. https://www.fda.gov/drugs/human-drug-compounding/compounding-and-fda-questions-and-answers.
