At Glaukos, our focus is to develop and lead the global ophthalmic market with novel therapies that advance the existing standard of care, and enrich the lives and treatment alternatives for patients worldwide.
Solutions For Healthcare Providers
Corneal Health Solutions
The iLink® Procedure
Transforming the Standard of Care for Progressive Keratoconus
iLink® corneal cross-linking is an innovative therapy that has transformed the treatment of progressive keratoconus. This minimally invasive outpatient procedure uses Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) and Photrexa® (riboflavin 5’-phosphate ophthalmic solution) riboflavin eye drops, combined with ultraviolet light from the KXL system to1:
- Create new corneal collagen cross-links
- Shorten and thicken collagen fibrils
- Stiffen and strengthen the cornea
[Beshtawi2013/p451/col2/para1/lines10-14; p452/col1/para1/lines3-6; col2/para1/lines10-11; lines23-27]
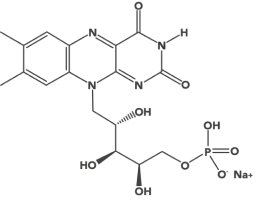
Riboflavin 5’–phosphate Sodium
Learn how iLink® corneal cross-linking can be used to slow or halt the progression of keratoconus and helps preserve vision in your patients.
View InfoExplore Patient Resources for Keratoconus Treatment Options
View Patient WebsiteFind a Physician Performing the iLink® Procedure
Go to Physician Locator
Market Access Support
Looking for Insurance
Reimbursement
Information?

Every patient deserves access to procedures that can help preserve their vision. iLink® corneal cross-linking helps patients avoid sight-threatening disease progression. That’s why GPS (Glaukos Patient Services) provides strategic and trustworthy market access solutions for all Glaukos procedures and products in glaucoma, corneal health, and retinal disease care.

Contact Us Today
to Integrate the iLink® Procedure Into Your
Practice
Explore additional information about iLink® in our digital brochure and learn how the procedure is transforming the standard of care for progressive keratoconus.
Request More Info
"*" indicates required fields
Reference
1. Beshtawi IM, O’Donnell C, Radhakrishnan H. Biomechanical properties of corneal tissue after ultraviolet-A-riboflavin crosslinking. J Cataract Refract Surg. 2013;39(3):451–462.
