Corneal Health Solutions
Get There in Time
We’re transforming the standard of care for patients with progressive keratoconus and other corneal ectatic conditions through our commitment to addressing important unmet clinical needs in corneal health.
With inspired innovation, a customer-centric focus, and prolific market access capabilities, we are in the constant pursuit of developing proven solutions in corneal health that empower eye care professionals to deliver optimal care for patients
What are the signs and symptoms of progressive keratoconus, and how can corneal cross-linking help?
Learn More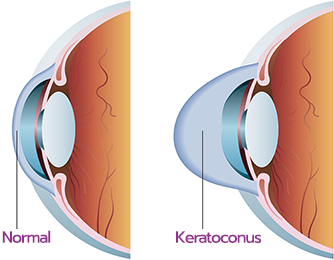
Resources for Your Practice
Our Comprehensive Support. Your Uncompromising Patient Care.
Integrating iLink® into your practice helps you expand care, and provide a wider range of benefits to many of your patients. As a trusted industry leader, we have the experience, tools, and training to make integration easy.


Market Access Support
Looking for Insurance
Reimbursement
Information?

Every patient deserves access to procedures that can help preserve their vision. iLink® corneal cross-linking helps patients avoid sight-threatening disease progression. That’s why GPS (Glaukos Patient Services) provides strategic and trustworthy market access solutions for all Glaukos procedures and products in glaucoma, corneal health, and retinal disease care.

Contact Us Today
to Integrate the iLink®
Procedure Into Your
Practice
Explore additional information about iLink® in our digital brochure and learn how the procedure is transforming the standard of care for progressive keratoconus.
Request
More Info
"*" indicates required fields

